Innov Ventilator
[EIT Success story 2021]
The Innov Ventilator addresses the ventilator shortage with a ventilator that is safe and easy to use, easy to manufacture and purely pneumatic – powered by air pressure alone. The ventilator can be used in emergency settings and is upgradable with common sensors and display technologies.
Innov Ventilator was one of three innovation projects the EIT and EIT Manufacturing selected as part of the EIT Crisis Response Initiative in 2020.

What are benefits for society and healthcare?
During the COVID-19 crisis, healthcare staff has had to face a shortage of the ventilator machines needed to save patients. The development of a purely pneumatic ventilator has the following advantages:
• No need for a power source, as it can be operated with a bottle of oxygen or mixture of gases.
• Faster development, with off-the-shelf components.
• Easier repair and maintenance, because no electronics are involved in the basic machinery.
• Quick to install and set up.
• Cost-efficient.
• Easy to use in emergency situations and outside hospital settings.
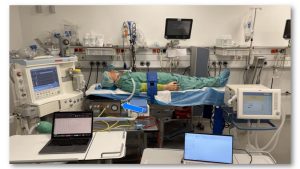
The Inno Ventilator is based on a purely pneumatic concept and is intended for several modes of pressure-controlled ventilation of patients with COVID-19 outside of the hospital setting. The device has been designed in line with regulatory and certification requirements, including usability, functional needs, and clinical requirements, and it is designated as a class IIb medical device.
The device has been tested in simulated real-life conditions, with very encouraging results. While pre-clinical trials have not yet been conducted, one of our partner developers, a medical doctor from Cologne, said that its flow and pressure is even better than much more expensive ventilators.
After testing the ventilator: “Once it is working, flow curves and pressure curves are better than €60,000 ventilators – that is impressive. We observe beautiful flow and pressure curves, even when the patient is coughing or breathing against the ventilation.
Dr. W. Wetsch (Univ. Hospital Cologne, partner)
What can you say about the team and the role of the EIT?
Thanks to the EIT Manufacturing community, the project benefited from a powerful innovation network, which facilitates collaboration with industry, research and education actors. EIT Manufacturing’s network allowed the project to build a consortium with all the interested partners: developers, industry, certification agents and clinicians.
The INNOV-Ventilator project is led by EIT Manufacturing’s Partner Tecnalia, together with a Spanish and German consortium consisting of TU Graz, Carl Reiner, WILD and University Hospital of Cologne from Germany, and Osakidetza and Biocruces Bizkaia Health Institute, Spain.
Thanks to a diverse team and EIT Manufacturing support, the project could get the solution off the ground quickly, undertaking design research and development.
The knowledge triangle has been integrated in the project with partners originating from all three areas. In this way, the final product has its basis in scientific evidence and clinical knowledge, and is integrated with the project’s business perspective to successfully reach the market. We have provided these education and training elements:
• Teaching personnel in hospitals how to use our novel device safely.
• Delivering educational content on safe ventilator usage to hospitals, by expanding the teaching efforts provided by Carl Reiner in collaboration with Technical University of Vienna.
• Addressing the business perspective with support from Carl Reiner, which has decades of expertise and experience in this market. The clinical organisations participating in this project also supported this effort by giving advice on certification issues.

